1. Intro
Understanding and manipulating the properties of materials to create new or improved products, encompasses
- Chemistry
- Physics
- Engineering
- Biology
How to? Understand the basics, dive deeper into material properties, learn about material processing, explore material characterization, apply to real-life application
1.1. Fundamental Principle
Structure influence the property, in different level (atomic, microscopic, macroscopic)
Properties and performance could be optimized
1.2. Goal
Developing new material
Improving existing material
Cost reduction
1.3. Question
How to design materials with specific properties
How to be more environmentally friendly
2. Basics
Main type:
- Metal, like steel, aluminum, copper; used in construction, transportation, electronics
- Ceramic: hard, brittle, have high melting points; used in construction, kitchenware, electronics
- Polymers: large molecules made up of repeating units. Natural like rubber or synthetic like nylon.
- Composites: made from two or more different materials, to achieve combined properties. Fiberglass and carbon fiber
- Semiconductors
Atomic structure and bonding:
- Structure: nucleus and electrons. The arrangement of electrons determines how atom interact and bond
- Bonds:
- Metallic bonds: in metal, electrons are shared in a ‘sea of electrons’
- Ionic bonds: between metal and non-metal, involving transfer of electrons
- Covalent bonds: in molecules and networks,
- Van der Waals bonds: weak bonds
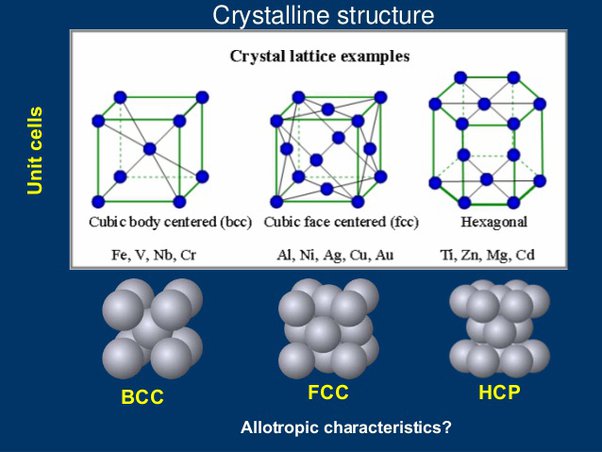
Crystallography: arrangement of atoms in crystalline solids
Crystal lattice (晶格), the unit cell is the smallest repeating unit in a lattice
Common structure
Face-centered cubic (FCC)
Body-centered cubic (BCC)
Hexagonal Close-Packed (HCP)
Xray diffraction: could be used to determine the arrangement of atoms
Imperfections in solids
- Points defects
- Dislocation
- Grain boudaries
- Surface defects
3. Material Properties
Mechanical properties, behavior under mechanical loads
Stress: $\sigma=\frac{F}{A}$
Strain: deformation caused by stress $\epsilon=\frac{\Delta L}{L_0}$
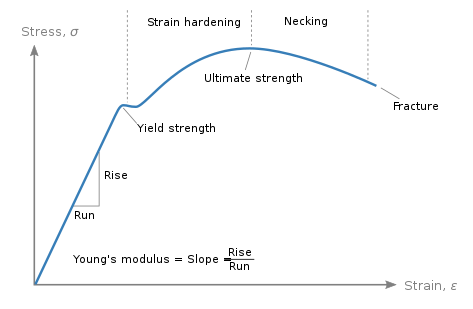
Elasticity: the ability to return to original shape after deformation
Plasticity: permanent deformation under load. Beyond the yield point, material deforms
Toughness: ability to absorb energy before failure, the area under the curve
Hardness: resistance to scratching
Fatigue: failure under cyclic loading
Creep: slow, time-dependent deformation
Thermal Properties
- Thermal Expansion, change in dimension with temperature
- Thermal conductivity: ability to conduct heat
- Specific heat: amount of heat required to raise the temperature of a unit mass by 1 C
- Thermal Diffusivity: the speed of heat conduction
Electrical Properties
- Electrical conductivity
- Resistivity
- Dielectric, behavior in electric field, important for insulator and capacitors
- Semi conductivity
Optical Properties
- Absoption
- Refection
- Refraction
- Transmission
- Refractive index
4. Material Processing
Goal: transform raw materials into final products with desired properties and characteristics
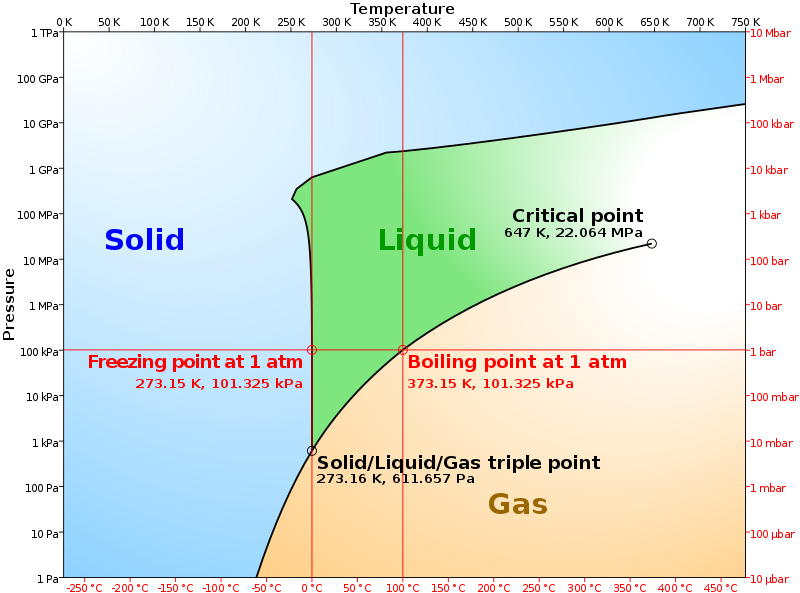
Phase diagrams
- A graphical representation that shows the phases of a material as a function
- Phase stability
- Lever rule
- Eutectic and peritectic points (共晶点、包晶点)
- Application: used to design alloy (合金), study material behavior under different condition
Heat Treatment
- Controlled process used to alter the physical properties of a material, usually metal
- Annealing: heating then slowly cooling
- Reduce hardness
- Increase ductility
- Relief internal stress
- Quenching: rapid cooling
- Increase hardness, strength
- Tempering: after quenching, heating to temp below the melting point
- Adjust hardness, strength and toughness
- Annealing: heating then slowly cooling
- Application: improve mechanical properties, relieve internal stresses
Material synthesis and Fabrication
- Synthesis: creation from simpler substances
- CVD: chemical vapor deposition: reaction produce a solid material
- Sputtering: ejecting material as thin film
- Sol-Gel process: from small molecules
- Fabrication: turning materials into parts
- Machining: for desired shape
- Casting(模型)
- Additive manufacturing
4.1. Question
Lever rule: determine the amount of each phase present in a two-phase system
Eutectic points: a point where three phase coexist in equilibrium at specific temperature and condition
Peritectic point: a point where three phase coexist but one of the solid phase react with the liquid phase form the other phase
Quenching: not enough time for atom to move into equilibrium, leading to disordered structure
Annealing: more ordered and stable structure
- Ordered structure are easier for movement
Internal stress? Not a single force, but a distribution of forces across an area
5. Material Characterization Technique
Help researcher and engineer understand material properties, structure, composition and performance.
Microscopy: visualize the micro and nano structure of materials
- Optical: visible light and lens, good for larger feature
- Limited resolution: 200nm
- Electron: electron beam instead of light
- SEM: 3d images of surface, with resolution in nm range
- TEM: very high resolution, sub nm
- Scanning probe microsopy
- AFM: atomic-level resolution
Spectroscopy:
- X-ray, for elemental analysis
- Infrared: molecular vibration
- Raman: studying crystal structure
- Nuclear Magnetic Resonance: study local magnetic field
6. Plan
6.1. Step 1: Understand the Basics
- Introduction to Materials Science: Learn about the different types of materials (metals, ceramics, polymers, composites, and semiconductors) and their properties.
- Atomic Structure and Bonding: Understand how atoms bond to form solids and how this affects the material’s properties.
- Crystallography: Study the arrangement of atoms in crystalline solids and learn about common crystal structures.
- Imperfections in Solids: Learn about various types of defects in materials and their impact on material properties.
6.2. Step 2: Dive Deeper into Material Properties
- Mechanical Properties: Understand stress, strain, elasticity, plasticity, toughness, hardness, and other mechanical properties of materials.
- Thermal Properties: Study how materials respond to changes in temperature, including thermal expansion, conductivity, and specific heat.
- Electrical Properties: Explore the electrical conductivity, resistivity, and dielectric properties of materials.
- Optical Properties: Learn about how materials interact with electromagnetic radiation, including absorption, reflection, refraction, and transmission.
6.3. Step 3: Learn About Material Processing
- Phase Diagrams: Understand phase diagrams and how they are used to predict the phase stability of different materials.
- Heat Treatment: Study how heat treatment can alter the properties of materials, particularly metals.
- Material Synthesis and Fabrication: Learn about various methods used to synthesize and fabricate materials.
6.4. Step 4: Explore Material Characterization Techniques
- Microscopy: Understand different microscopy techniques (e.g., optical, electron, scanning probe) used to study materials at the micro and nanoscale.
- Spectroscopy: Learn about various spectroscopy techniques used to study the composition and properties of materials.
- Mechanical Testing: Familiarize yourself with different mechanical testing methods to evaluate material properties.
6.5. Step 5: Apply Your Knowledge to Real-Life Applications
- Material Selection: Learn how to choose the appropriate material for a specific application based on its properties.
- Failure Analysis: Understand how to analyze failed components to determine the cause of failure and prevent future incidents.
- Sustainability and Lifecycle Analysis: Consider the environmental impact of materials and learn about sustainable material practices.